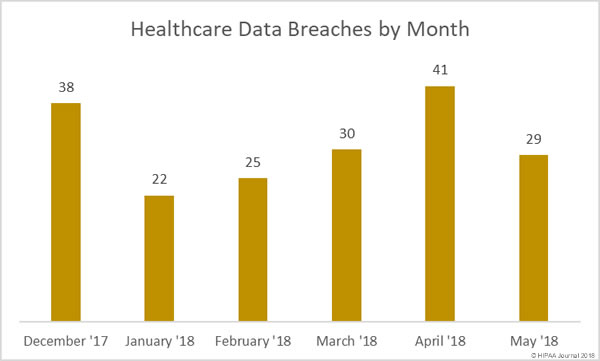
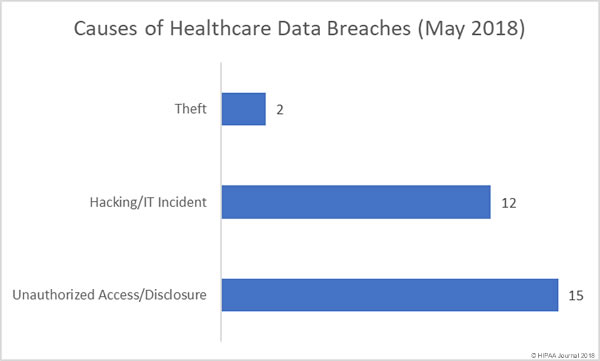
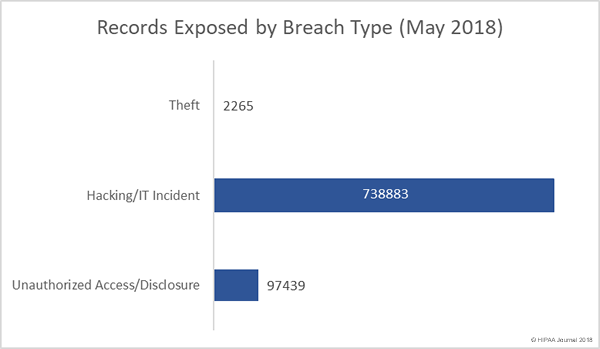
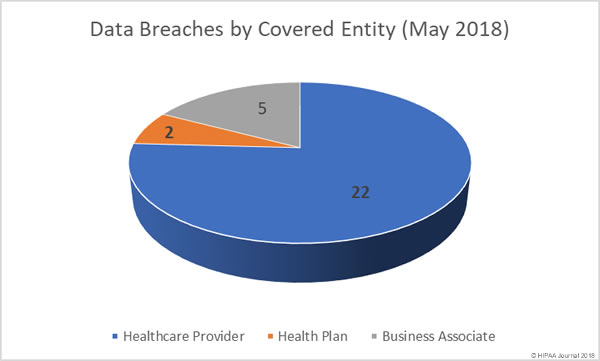
Following the alleged inappropriate accessing of patient health records by employees, Washington Health System has taken the decision to suspend several employees while the privacy breach is investigated.
While it has not been confirmed how many employees have been suspended, Washington Health System VP of strategy and clinical services, Larry Pantuso, issued a statement to the Observer Reporter indicating around a dozen employees have been suspended, although at this stage, no employees have been fired for inappropriate medical record access.
The privacy breaches are believed to relate to the death of an employee of the WHS Neighbor Health Center. Kimberly Dollard, 57, was killed when an out of control car driven by Chad Spence, 43, rammed into the building where she worked. Spence and one other individual were admitted to the hospital after sustaining injuries in the accident.
Pantuso did not confirm that this was the incident that prompted the employees to access patients’ medical records, although he did confirm that the alleged inappropriate access related to a “high profile case.”
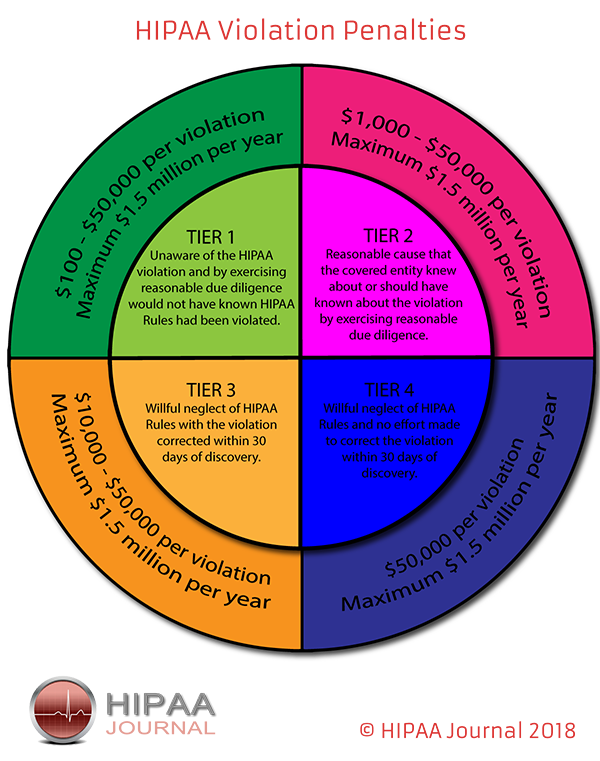
The accessing of medical records without any legitimate work reason for doing so is a violation of the Health Insurance Portability and Accountability Act (HIPAA). HIPAA only permits the accessing of PHI by employees for treatment, payment, or healthcare operations.
Any healthcare employee discovered to have violated HIPAA Rules faces disciplinary action which can involve suspension, termination, loss of license and, potentially, criminal charges.
There have been several recent cases where employees have been fired snooping on the medical records of high profile patients.
In February 2018, 13 employees of the Medical University of South Carolina were fired for HIPAA violations after they accessed the medical records of patients without authorization, many of whom accessed the medical records of high profile patients.
One of the most recent actions taken against a healthcare employee for a HIPAA violation was taken by the New York nursing board’s Office for Professional Discipline. Martha Smith-Lightfoot was provided with a list of patients prior to leaving her employment at University of Rochester Medical Center (URMC) to take up a new position at Greater Rochester Neurology. Smith-Lightfoot provided that list to her new employer and patients were contacted in an attempt to solicit business.
Smith-Lightfoot signed a consent order with the nursing board admitting the violation and had her license to practice suspended for one year, received a stayed suspension for another year, and three years of probation when she returns to practice.
Snooping on medical records is likely to be discovered as logs are created when health records are accessed. Those logs are periodically checked and if inappropriate PHI access is discovered it is likely to result in termination and will make it hard to obtain future employment in healthcare.
The post Washington Health System Suspends Several Employees for Inappropriate PHI Access appeared first on HIPAA Journal.