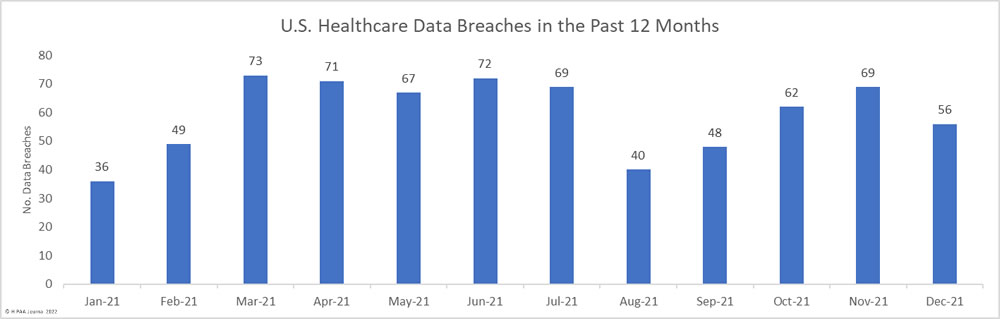
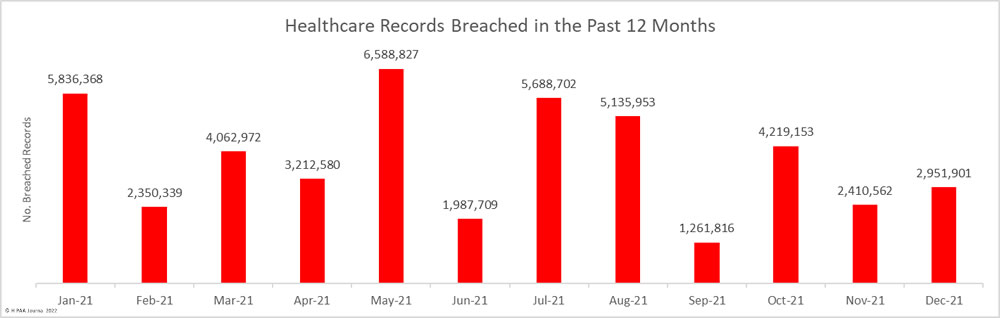
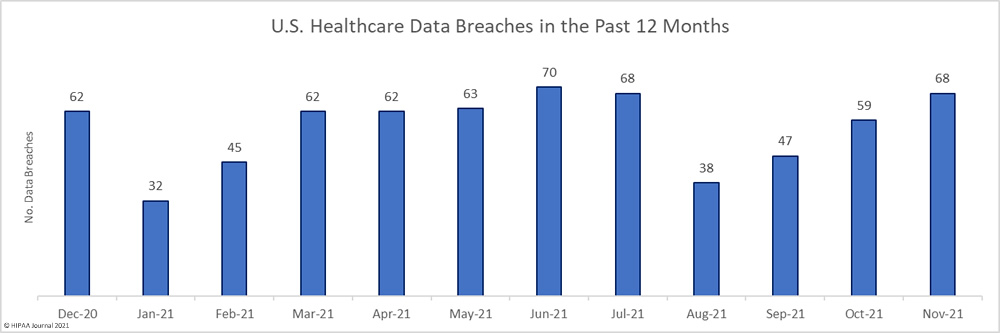
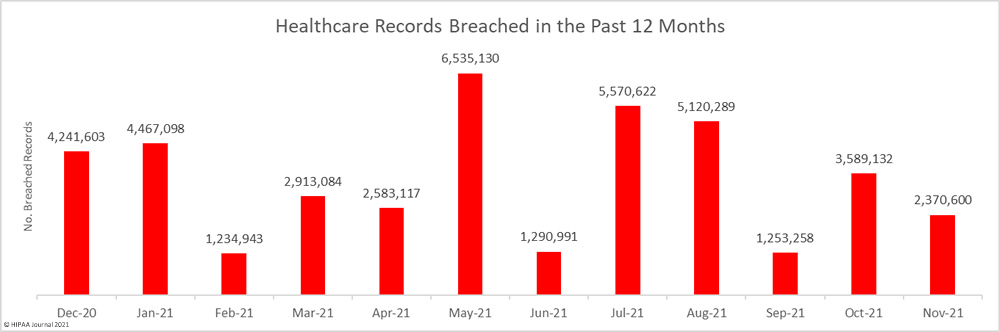
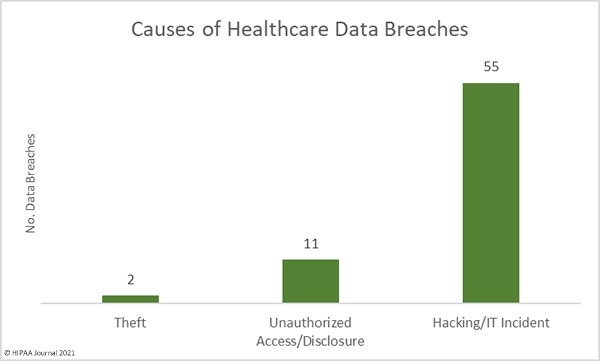
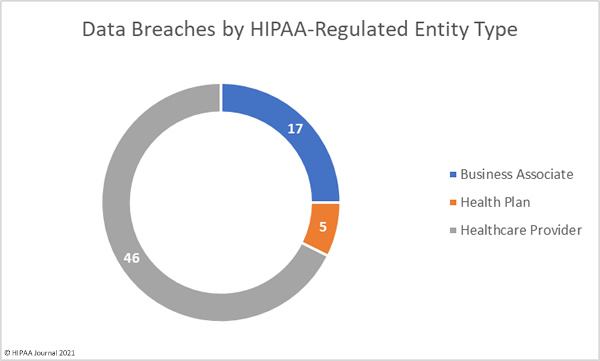
The first settlement of 2022 to resolve a healthcare data breach has been announced by New York Attorney General Letitia James. The Ohio-based vision benefits provider EyeMed Vision Care has agreed to pay a financial penalty of $600,000 to resolve a 2020 data breach that saw the personal information of 2.1 million individuals compromised nationwide, including the personal information of 98,632 New York residents.
The data breach occurred on or around June 24, 2020, and saw unauthorized individuals gain access to an EyeMed email account that contained sensitive consumer data provided in connection with vision benefits enrollment and coverage. The attacker had access to the email account for around a week and was able to view emails and attachments spanning a period of 6 years dating back to January 3, 2014. The emails contained a range of sensitive information including names, contact information, dates of birth, account information for health insurance accounts, full or partial Social Security numbers, Medicare/Medicaid numbers, driver’s license numbers, government ID numbers, birth/marriage certificates, diagnoses, and medical treatment information.
Between June 24, 2020, and July 1, 2020, the attackers accessed the account from multiple IP addresses, including some from outside the United States and on July 1, 2020, the account was used to send around 2,000 phishing emails to EyeMed clients. The EyeMed IT department detected the phishing emails and received multiple inquiries from clients querying the legitimacy of the emails. The compromised account was then immediately secured.
The subsequent forensic investigation confirmed the attacker could have exfiltrated data from the email account while access was possible but could not determine if any personal information was stolen. Affected individuals were notified in September 2020 and were offered complimentary credit monitoring, fraud consultation, identity theft restoration services.
The Office of the New York Attorney General investigated the security incident and data breach and determined that, at the time of the attack, EyeMed had failed to implement appropriate security measures to prevent unauthorized individuals from accessing the personal information of New York residents.
The email account was accessible via a web browser and contained large quantities of consumers’ sensitive information spanning several years, yet EyeMed had failed to implement multifactor authentication on the account. EyeMed also failed to implement adequate password management requirements for the email account. The password requirements for the account were not sufficiently complex, only requiring a password of 8 characters, when it was aware of the importance of password complexity as the password requirements for admin-level accounts required passwords of at least 12 characters. EyeMed also allowed 6 failed password attempts before locking out the user ID. EyeMed had also failed to maintain adequate logging of email accounts and was not monitoring email accounts, which made it difficult to identify and investigate security incidents. It was also unreasonable to retain consumer data in the email account for such a long period of time. Older emails should have been transferred to more secure systems and be deleted from the email account.
State attorneys general have the authority to impose financial penalties for HIPAA violations and it would have been possible to cite violations of HIPAA; however, New York only cited violations of New York General Business Law.
Under the terms of the settlement, EyeMed is required to pay a financial penalty of $600,000 and must implement several measures to improve security and prevent further data breaches. Those measures include:
- Maintaining a comprehensive information security program that is regularly updated to keep pace with changes in technology and security threats
- Maintaining reasonable account management and authentication, including the use of multi-factor authentication for all administrative or remote access accounts
- Encrypting sensitive consumer information
- Conducting a reasonable penetration testing program to identify, assess, and remediate security vulnerabilities
- Implementing and maintaining appropriate logging and monitoring of network activity
- Permanently deleting consumers’ personal information when there is no reasonable business or legal purpose to retain it.
“New Yorkers should have every assurance that their personal health information will remain private and protected. EyeMed betrayed that trust by failing to keep an eye on its own security system, which in turn compromised the personal information of millions of individuals,” said Attorney General James. “Let this agreement signal our continued commitment to holding companies accountable and ensuring that they are looking out for New Yorkers’ best interest. My office continues to actively monitor the state for any potential violations, and we will continue to do everything in our power to protect New Yorkers and their personal information.”
The post New York Fines EyeMed $600,000 for 2.1 Million-Record Data Breach appeared first on HIPAA Journal.